
To ensure complete denaturation of the proteins, samples are usually boiled at 95 ☌ for 5–10 min before loading. The final step is to mix the samples with a loading buffer or sample treatment buffer composed of (a) glycerol to increase the density of the sample facilitating its loading on the gel, (b) a coloring dye to make the sample visible (bromophenol or Coomassie Blue), (c) the agents reducing disulfide bonds (β-mercaptoethanol or dithiothreitol, DTT), (d) SDS in case of denaturing conditions, and (e) Tris-HCl. so as to ensure that the total protein amount in each sample under analysis is equivalent. It is also important to have an idea of the protein concentration in the extracted sample using a suitable method It is useful to know the protein concentration in the lysates to control the amount loaded on the gel and to allow comparison between experiments. As the cell lysis leads to the release of proteases and phosphatases, the samples must be kept at 4 ☌ and protease inhibitor cocktail must be used to prevent any further protein degradation. Although these agents are less harsh and less denaturing and keep the proteins relatively intact, the protein yield is comparatively low especially from subcellular organelles and membranes. The other agents used for sample preparation are nonionic detergents which generally include Triton X-100, Nonidet P-40, Tween, etc. The viscosity of the sample treated with ionic buffers is also higher due to the release of the chromatin and thus may need sonication. The ionic buffers are supposed to provide higher yield of proteins as compared to the nonionic buffers however, this may lead to the loss of some functional properties of proteins. Sample denaturing buffers contain the powerful anionic detergent sodium dodecyl sulfate (SDS), which linearizes the proteins, and a reducing agent such as 2-mercaptoethanol to break disulfide bonds. Protein samples from cultured cells can be extracted either by direct dissolution in a denaturing buffer or by homogenization in a buffer containing protease inhibitors. The first step for western blotting is sample preparation. Although the western blotting protocol for specific purpose can be optimized, here we discuss step-by-step, the general principle and protocol followed for western blotting procedure in virology. This technique is very useful to detect the presence of a given protein in a sample, to assess posttranslational modifications such as phosphorylation and to characterize protein complexes. An appropriate substrate is then added to the enzyme and together they produce a detectable product such as a chromogenic or fluorogenic precipitate on the membrane for colorimetric or fluorometric detection, respectively. The transferred protein is then complexed with an enzyme-labeled antibodyĪs a probe. Next, the membrane is blocked to prevent any nonspecific binding of antibodies to the surface of the membrane. Following electrophoresis, the separated molecules are transferred or blotted onto a second matrix, generally a nitrocellulose or polyvinylidene difluoride (PVDF) membrane.


The first step in a Western blotting procedure is to separate the macromolecules using SDS-polyacrylamide gel electrophoresis (SDS-PAGE in 1979 and the name was given by Burnette in 1981 to match similar techniques used for detection of DNA in Southern blotting and RNA in Northern blotting. The technique was initially described by Towbin et al. Is used to specifically detect the antigen) is a widely used analytical technique to detect specific proteins in a given sample of tissue homogenate or extract. The western blot (often called as the protein immunoblot because an antibody
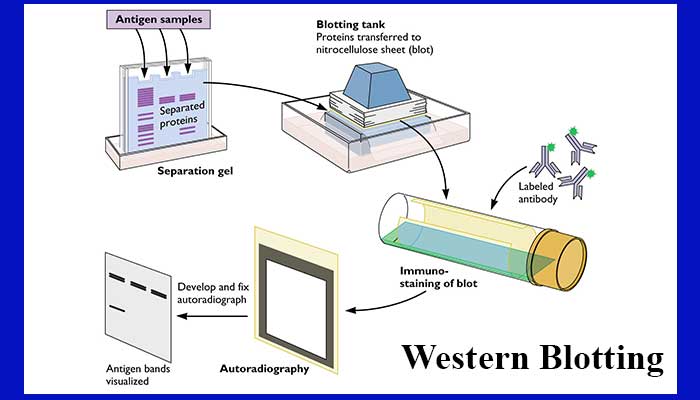
Of biological samples from a gel on to a membrane and their subsequent detection on the surface of the membrane. The term “blotting” refers to the transfer


 0 kommentar(er)
0 kommentar(er)
